Ozurdex Injection
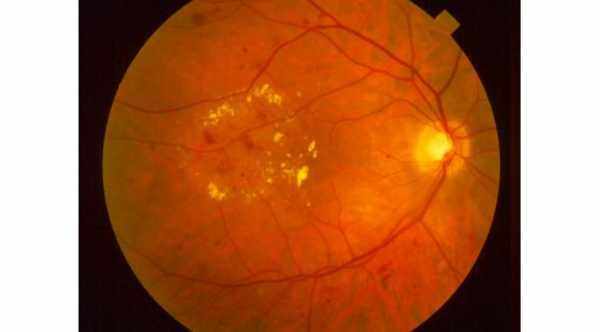
Ozurdex is a prescription medicine used for the treatment of diabetic macular edema, macular edema (swelling of the macula) as a result of branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO), and noninfectious uveitis (inflammation of the uvea) affecting the posterior segment of the eye. Ozurdex aims to regain some of the lost vision that may have been caused by the macular edema.
Ozurdex is an intravitreal Steroid implant that is injected into the eye. It’s a sustained-release implant that contains dexamethasone. Dexamethasone is a corticosteroid medicine that is being used to block chemical pathways which lead to swelling of the retina, leakage from the blood vessels in the retina, and inflammation. It is the first, and currently the only biodegradable dexamethasone implant in injectable form.
How does Ozurdex Injection Work?
It uses an advanced Novadur drug delivery technology to create a tiny rod-shaped implant combining a biodegradable material with its active ingredient which is dexamethasone. While implanted inside the eye, the tiny implant slowly dissolves with the help of the vitreous gel in the eye, releasing the corticosteroid drug. Since it is biodegradable, it doesn’t have to be removed after the medication is released.
Most Commen Side Effects of Ozurdex
1. For the treatment of Diabetic Macular Edema
Increased eye pressure, blood spot in the conjunctiva, swelling of the conjunctiva, floaters, reduced visual acuity, dry eyes, foreign body sensation, vitreous detachment and opacities, retinal aneurysm (dilation of retinal arteries), retinal tear, corneal erosion, inflammation of the conjunctiva/cornea/anterior chamber, drooping of the eyelid, and bronchitis.
2. For the treatment of Retinal Vein Occlusion and Uveitis
Increased eye pressure, blood spot in the conjunctiva, redness on the eye, headache, eye pain, cataract, vitreous detachment, and ocular hypertension.
Possible Complications of Ozurdex Injection
• Intravitreal injections such as with Ozurdex are associated with endophthalmitis (severe eye infection involving the interior of the eye).
• Retinal detachments may also occur.
When Not to use Ozurdex Intraocular Injection
• It is contraindicated for patients suffering from glaucoma that has a cup-to-disc ratio of more than 0.8.
• It is contraindicated for patients with torn or ruptured posterior lens capsule.
• It is contraindicated for patients having allergy to any of its components.
• It is not recommended for patients who had a history of ocular herpes simplex, or patients currently having infections or diseases in the eye or eye area. Most viral diseases of the eye, vaccinia, varicella, fungal diseases and mycobacterial infections are contraindications for Ozurdex injection.
Patient Education
• It is advised not to drive or operate cars immediately after receiving an Ozurdex injection as a temporary blurring of vision may occur.
• The patient may be at risk for potential complications days after Ozurdex injection. It is vital to immediately report any signs of redness, sensitivity to light, pain, or changes in vision to your eye doctor.
• A cataract may occur after repeated injections with Ozurdex. In a related clinical study, 68% of patients with natural lenses developed cataracts after Ozurdex injections. If a cataract occurs and the vision decreases, a cataract surgery will be required to remove the opacified lens and to restore vision.
• It is important to discuss your condition and possible benefits of Ozurdex with your eye doctor. Each case is different and results may vary.