Geographic Atrophy Treatment with APL-2
What is Geographic Atrophy?
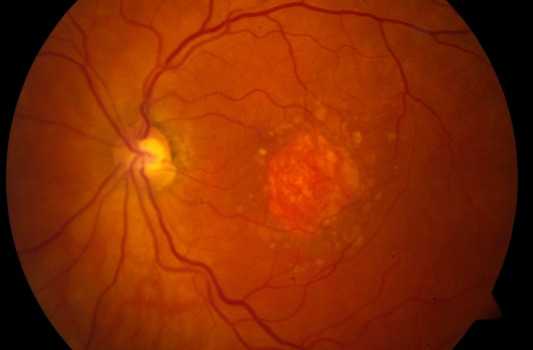
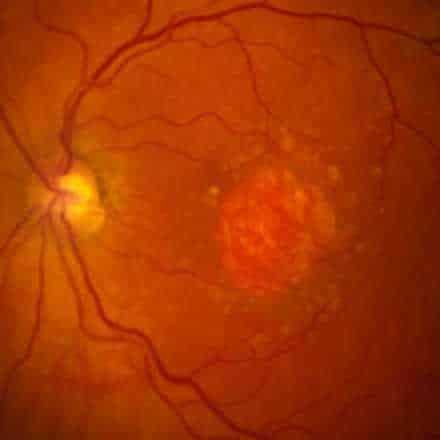
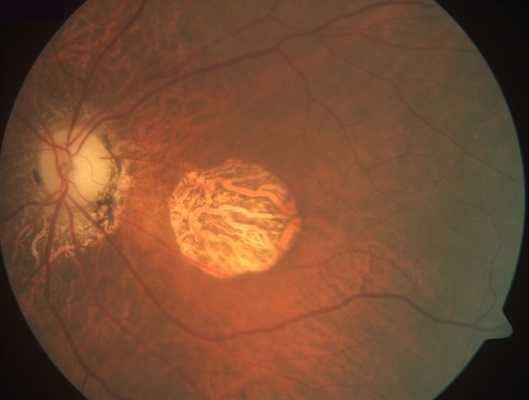
Geographic Atrophy (GA) is an advanced form of Dry Age-Related Macular Degeneration (AMD) that affects about 5 million persons worldwide. GA is a chronic degenerative disease that is characterized by a progressive loss of regions of the retina (retinal pigment epithelium (RPE), photoreceptors, and choriocapillaris) causing irreversible visual loss. The permanent loss of visual function is usually bilateral.
The exact cause of GA is unknown, though genetic and environmental factors are considered to contribute significantly. Several studies noted the following risk factors: family history of AMD, Age, smoking history (active and former smokers), use of thyroid hormones or antacids, coronary heart disease, and lens opacities or previous cataract surgery.
The prognosis is poor due to unavailability of treatment. Currently there are no effective preventive measures or any approved medical or surgical treatments that can stop progression or reverse the visual loss. However, several products are under clinical trials.
What is APL-2?
APL-2 is a complement inhibitor drug that activates at the level of C3, designed to halt the progression of geographic atrophy. It is a synthetic cyclic peptide combined to a polyethylene glycol polymer that can inhibit all pathways of activation to complement inflammatory system—classical, alternative and lectin.
APL-2 may provide the following benefits:
Prevent or mitigate the progression of retinal cell death – The phase 2 trial of APL-2 in patients with GA resulted in a significant reduction in GA lesion growth rate over 12 months.
Possible application to all patients suffering with GA regardless of the complement pathway causing the disease – APL-2 is designed to address all 3 principal complement pathways, giving the potential treatment in a broad patient population.
Every other month administration – In the phase 2 clinical trial, the primary endpoint was met in both monthly and every other month treatment administration.
On August 24, 2017 Apellis Pharmaceuticals announced that the APL-2 has met its primary endpoint in a phase 2 study. The study involved 246 patients with Geographic Atrophy which is associated with age-related macular degeneration.
The APL-2 was injected intravitreally on a monthly basis and has shown a 29% reduced GA lesion growth rate compared to sham, while intravitreal injections given every other month reduced the rate by 20%.
During the second 6 months of study, the effect of APL-2 appears to increase by almost half. The reduction in the growth rate was 47% for the monthly administration, and 33% with the every other month administration.
The Phase 2 sham-controlled clinical trial was conducted at 40 clinical facilities located in the US, New Zealand, and Australia. The primary endpoint was based on the GA lesion size changes within the 12-month trial, compared to sham.
The most common adverse reactions during the treatment were related to the injection procedure. A high incidence of Wet AMD was noted especially in patients with history of wet AMD in the fellow eye. The affected eyes were treated with anti-VEGF. The vision was not affected and there was no effect on the outcome of the clinical trial.
After 12 months of treatment, the patients were monitored for 6 more months. Apellis Pharmaceuticals plans to initiate Phase 3 on the second half of 2018.