MicroPine for Progressive High Myopia
Eyenovia Inc. announced that its MicroPine therapeutic program for the treatment of progressive myopia will advance to Phase III clinical development, which is planned to start in 2019. On February 21, 2018, the company confirmed that it received a clear feedback that only one Phase III pivotal study will be required for registration, as discussed with the U.S. Food and Drug Administration (FDA).
Dr. Douglas Fredrick, a pediatric ophthalmologist has been appointed to Eyenovia’s Scientific Advisory Board and Myopia Program Steering Committee to guide the company in advancing its MicroPine therapeutic program for myopia. Dr. Douglas Frederick is well-experienced in myopia prevention and pediatric cataracts and is currently a clinical professor of Ophthalmology and Pediatrics at Lucile Packard Children’s Hospital.
With a strong Scientific Advisory Board of experts, Eyenovia believes that its microdosing technology will be further developed and will create a big impact in the treatment of ocular diseases.
About Progressive High Myopia
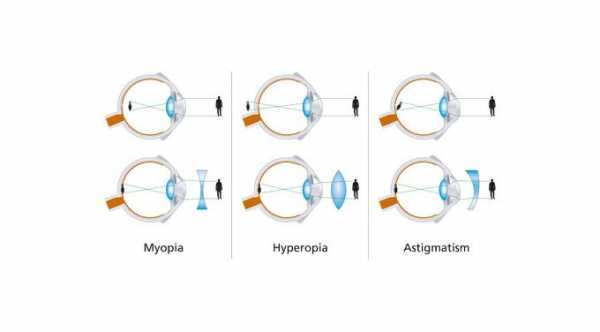
Myopia or nearsightedness is an eye condition where light rays passing into the eye are focused in front of the retina, instead of being focused on the retina where light is converted to neural signals and are then interpreted by the brain for visual recognition.
Progressive myopia is characterized by a refractive error of more than -6.00D with an axial length of more than 26mm. It is degenerative and progressive that may involve stretching of the retina, choroid, and sclera.
About MicroPine therapeutic program
MicroPine is Eyenovia’s proprietary microdose formulation of atropine for the treatment of progressive myopia. Results of the second phase 2 trial for MicroPine were recently published showcasing the success of its microformulation approach.
The treatment for progressive myopia is aimed towards slowing down the progress of the condition. Recent studies have shown that daily use of 0.01% atropine eye drops can slow down the progression of myopia with fewer side effects compared to higher doses. A rebound effect was also not observed after stopping the medication.
Other studies were conducted with different dose concentrations, and it was concluded that despite a relatively high rate of adverse effects, the use of atropine can be a sustainable and effective treatment for progressive myopia. Atropine’s role in the treatment of progressive myopia is not yet well established but it has been the standard of care for some countries.
The goal of myopia control is to slow the progression of myopia during the years where eye growth is most active, and thus the availability and low cost of atropine is a sustainable treatment option.
About Eyenovia
Eyenovia Inc. is a clinical stage specialty biopharmaceutical company developing ophthalmology products based on its patented piezo-dispersion and microdosing technology designed to deliver micro-therapeutics to the eye. It aims to transform the ocular drug delivery system for the treatment of a variety of eye diseases.
Eyenovia Inc. uses piezo-print electronics and microfluidics to create microdrops and high-precision microdosing. Eyenovia’s microdrops are gentler to the eye while preventing delivery of unnecessary extra doses and preservatives that may be associated with pain, redness, and irritation.
This smart technology reduces overdosing from conventional eye drops to about 80%. The microdosing technology can deliver 6-8 µL volumes compared to 30-50 µL from conventional eye droppers.