Gevokizumab showed promises to treat uveitis in patients with Behcet Disease
Gevokizumab (Xoma) is a monoclonal antibody that binds to interleukin-1β (IL-1β). This interleukin is a pro-inflammatory cytokine that play major role in the inflammatory non-infectious uveitis that occurs in many systematic diseases such as behcets disease.
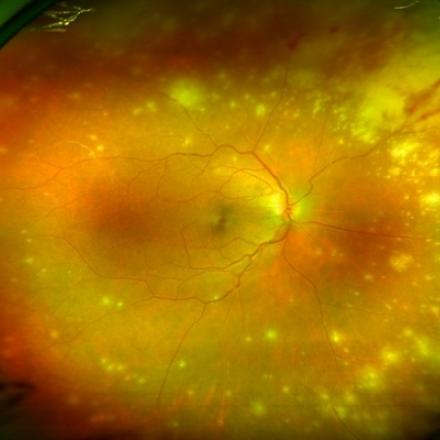
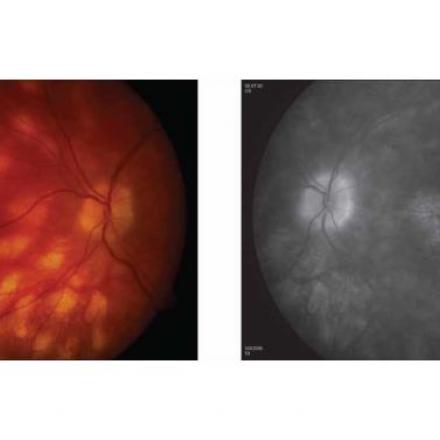
Uveitis is an inflammation of the uvea part of the eye which is the middle vascularized layer and consists mainly of choroid. When gevokizumab binds to interleukin-1β, it inhibits the interleukin -1 receptor, thereby inhibiting cellular reactions and events that are responsible for uveitis.
Symptoms of uveitis are decreased and blurred vision, ocular pain, redness, eye floaters and photophobia or light sensitivity. Untreated uveitis can lead to complete blindness.
Uveitis in behcet disease is characterized by being severe and with recurrent attacks and without immediate and proper treatment, complications can occur such as retinal veins and retinal arteries obstruction, ischemic retina, vitreous hemorrhage, glaucoma and retinal detachment.
Corticosteroid and immunosuppressive medications are the main modalities of treatment of behcet disease but there are associated with short term and long term complications.
A Phase 2 clinical trial on using this medication to treat uveitis in 7 patients with behcets disease was successfully completed by XOMA. With a single dose and with discontinuation of commonly used immunosuppressive drugs such as azathioprine and cyclosporine, all these seven patients showed rapid improvement of uveitis and improvement in their visual acuities.
Recurrent uveitis in these patients was responded again to retreatment with a single dose. No side effects related to the drug were reported during the trial.
On October, 3, 2012, XOMA announced that the enrollment in a Phase 3 clinical trial was open. This clinical trial will determine the efficacy and the safety of this medication to reduce the risk of recurrent of uveitis. Patients will be randomized to receive monthly for twelve months either doses of placebo or gevokizumab.